Science Guides Us During the Crisis for the New Coronavirus
By Mauricio Rodriguez, Ph.D.
Scientific Affairs Director CropLife Latin America
April 21st, 2020
 We are navigating uncharted waters, at least for current generations, in which globalization may be at the center of cases like that of the current SARS-CoV2 virus pandemic that causes the disease known as COVID-19, provoking unprecedented physical, psychological, social and economic damage1. At the same time that today's technologies have given us access to information that has helped the whole world take notice, relatively soon, about the high risk of this disease, we can find in science the solutions and responses to situations like this that seem to overwhelm our capacity and resilience as the dominant species of the planet. For it is precisely science that is called upon to offer us answers, solutions, and reaffirmation in the human condition. Let us look at some examples of how science guides us and is helping us overcome this situation. From supporting decision-making by political leaders and institutions that are based on mathematical and epidemiological models of the contagion and the knowledge helping health personnel in clinics and hospitals, to the decisions we individually make in our daily lives to protect us, science is present. For the purpose of not extending this reading too much, let's look at just a few examples and divide them between scientific non-pharmaceutical and pharmaceutical intervention measures. Non-pharmaceutical measures include those such as confinement or physical separation among people, hand hygiene and the use of personal protective equipment such as masks or face-covering in public places. Below, we briefly explain the science behind these solutions.
We are navigating uncharted waters, at least for current generations, in which globalization may be at the center of cases like that of the current SARS-CoV2 virus pandemic that causes the disease known as COVID-19, provoking unprecedented physical, psychological, social and economic damage1. At the same time that today's technologies have given us access to information that has helped the whole world take notice, relatively soon, about the high risk of this disease, we can find in science the solutions and responses to situations like this that seem to overwhelm our capacity and resilience as the dominant species of the planet. For it is precisely science that is called upon to offer us answers, solutions, and reaffirmation in the human condition. Let us look at some examples of how science guides us and is helping us overcome this situation. From supporting decision-making by political leaders and institutions that are based on mathematical and epidemiological models of the contagion and the knowledge helping health personnel in clinics and hospitals, to the decisions we individually make in our daily lives to protect us, science is present. For the purpose of not extending this reading too much, let's look at just a few examples and divide them between scientific non-pharmaceutical and pharmaceutical intervention measures. Non-pharmaceutical measures include those such as confinement or physical separation among people, hand hygiene and the use of personal protective equipment such as masks or face-covering in public places. Below, we briefly explain the science behind these solutions.
Soap: A powerful weapon against infections
Let us look at the case of handwashing. Why do health experts insist that something as simple as soap can help us control an invisible pathogen like SARS-CoV2? This is how soap works: soap is a chemical substance that can be obtained naturally or synthetically, the molecular shape of which resembles a pin. These molecules have a hydrophilic head (which easily binds to water molecules) and a hydrophobic tail, which repels water and prefers to bind to fatty molecules. When soap molecules are suspended in water, they spontaneously bind together forming small bubbles called micelles, with the heads of molecules outward and tails inwards. These micelles in the soap and water mixture are responsible for trapping compounds inside or, in the case of coronavirus, organisms that have a lipid or fatty surface, such as food residues or the membrane of virus.
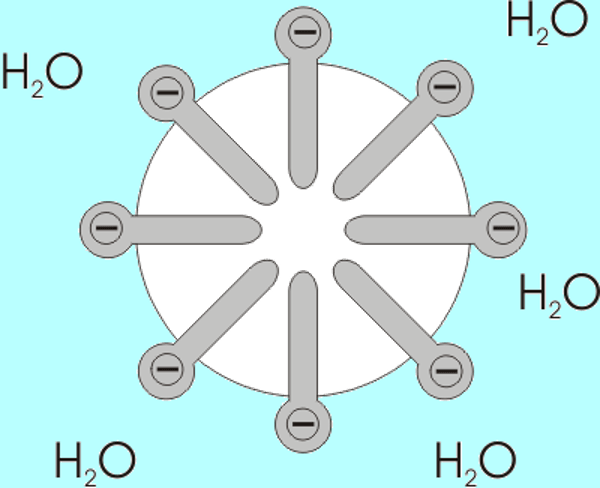
Soap molecules suspended in water. The hydrophilic head can be identified towards the water solution, and the hydrophobic tail pointing towards the center, away from the water.
(Source: Wikimedia, 2020. https://upload.wikimedia.org/wikipedia/commons/0/0d/Micelle.png)
Different viruses, such as SARS-CoV2, easily bind to the skin because the cells that make it up also have lipid membranes that attract each other. So when we efficiently wash our hands with soap and water, both micelles and molecules free of it trap or break down the lipid membrane of the virus, separating it from our skin or destroying it2.
Face masks and respirators also help prevent contagion
Although public policies in cases of epidemic or pandemic emergency suggest reserving the use of these devices to health personnel or infected patients, studies have shown that their use by uninfected healthy people also helps prevent the spread of respiratory virus diseases. Basically, these face covers are physical barriers that decrease by various percentages the likelihood (the risk) that infectious bioaerosols (saliva drops or mucus) that carry the virus will fall into our airways. There are different levels of protection according to the material and quality of these masks.

(Source: Emory University, 2020. https://www.ehso.emory.edu/documents/toolbox-training-respirator-protection.pdf)
A collaborative study between researchers in Hong Kong and the United States determined through a randomized clinical trial that the use of the most common type of surgical masks, along with hand washing, contributes to the decrease in influenza virus infection if these mitigation measures are performed early in the infection cycle3. Similarly, a study in the UK in 2013 showed that the use of surgical masks can significantly reduce exposure to infectious bioaerosols4. This April 2020, some of the Hong Kong researchers mentioned above published a new study reinforcing their previous findings on the effectiveness of masks to prevent coronavirus contagion5. These physical barriers serve as a complementary measure of risk mitigation, when we cannot avoid another type of intervention to limit exposure such as physical isolation.
Confinement or physical isolation: Epidemiological Models
The practice that is being made known as social estrangement, more correctly is called confinement or physical isolation, since we remain a functional part of society, although under certain restrictions. Governments are constantly weighing the enormous amount of information available and trying to solve as many questions as possible to make the best isolation decisions under the current circumstances: closing borders, like airports? Restrict mobility? Close schools and universities? These decisions are based on statistical and epidemiological scientific models that, although not extremely complex from a scientific point of view, are very difficult to build because of the large number of variables on which the information is not complete. We already have some of the basic parameters of the virus's behavior, such as how many more people can get it for each infected person; or how long it takes for an infection to resolve. But we still don't have much necessary epidemiological information, for example, how many asymptomatic people are infected?
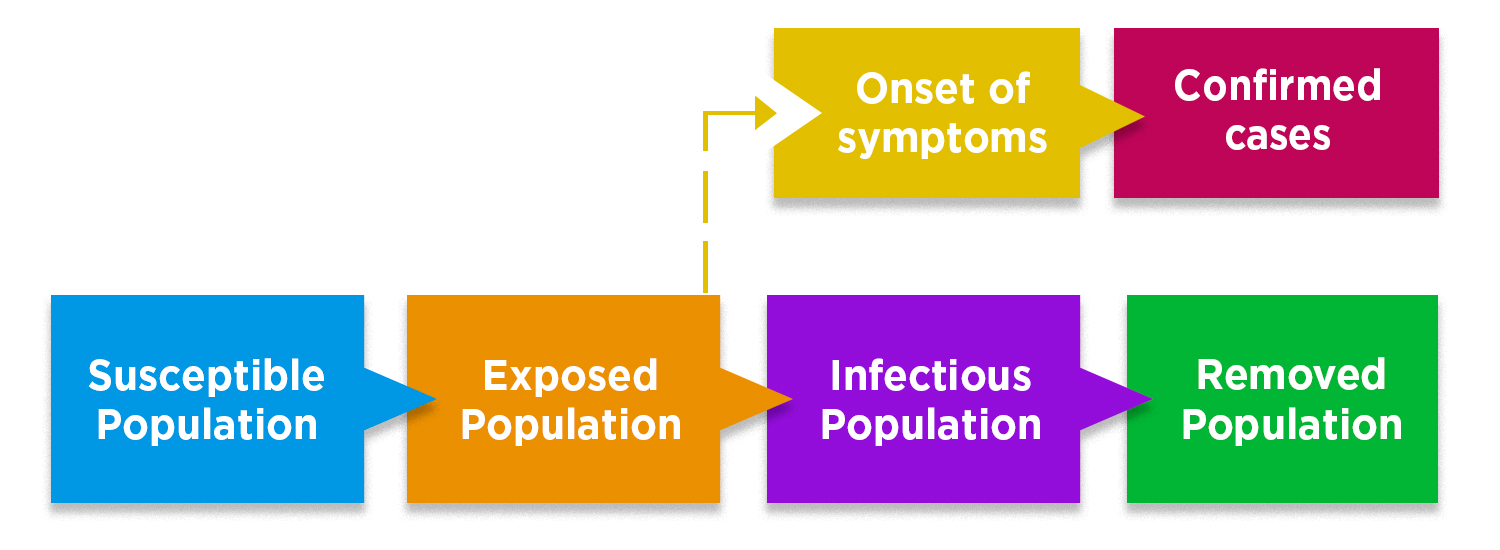
In this model, the population is divided in 4 main groups: En este modelo, se divide la población en 4 grupos principales: susceptible, exposed (no symptoms yet), infectious (with symptoms), y removed (isolated, recovered, or not infectious). Adapted from The Lancet Infectious Diseases, 2020. https://www.sciencedirect.com/science/article/pii/S1473309920301444
This is one of the main questions that has not yet been resolved because we would need serological data to verify that each asymptomatic person has antibodies to the virus. This is obviously very difficult to respond because 100% of the population would have to be assessed. So the models serve to seek an approximation of reality and make decisions with the most complete information available. Models are being used to understand how, for example, after establishing mobility restrictions as happened in China, the rate of infection outbreaks can be slowed6. Governments are clear that they cannot completely suppress the establishment of contagion once it has been established by multiple independent cases in the country, so measures seek to minimize the economic impact and overflow of the capacity of health services to treat those who become ill most seriously.
Medicines and Vaccines: Urgency and Safety
An important challenge that the current situation imposes on us is that the public wants science to produce in the very short term technological solutions that immediately curb the infection and severity of the disease, in other words, therapies and prophylactics. But the problem with this expectation imposed on science is the ignorance of just how science is done— with rigorous, methodical processes that take time and independent verification that validate both the effectiveness and safety of technologies. This is especially important in cases of medicines and vaccines, which must follow rigorous risk assessment protocols before being suitable for clinical use, especially if they are to be used for widespread use. There are numerous reports in both news and scientific media, on impressive advances in laboratory and clinical studies on medicines and vaccines that are expected to be available in the short term to deal with the emergency, but we must be smart and not ignore the scientific rigor on which risk assessment is based.
As in many other infectious pathologies, the first therapeutic defense recommendations focus on the use of acetaminophen, to control fever, and ibuprofen, in those cases with greater severity that require their anti-inflammatory effects7. This therapy is surely sufficient in non-critical cases; for the most serious, other types of antiviral therapies are being developed as described below.
One thing that needs to be clear is that although several companies are on the run to develop a vaccine, it would not be available in the short term. At best, it could be used preemptively in case the same virus re circulates in the second half of 2021, although it is likely to take longer8. Although it seems a little late, these vaccines are developing very quickly. Vaccines typically develop from viruses grown in cell cultures, which involves other variables and protocols that require several years. The first vaccine for COVID-19 already in clinical studies was developed by the U.S. National Institute of Allergy and Infectious Diseases (NIAID), in collaboration with Moderna Inc. This vaccine has been developed from a genetic platform called messenger RNA that was initially developed to fight MERS (Middle East Respiratory Syndrome) coronavirus, but was quickly adapted for COVID-19. This vaccine is already in phase I clinical trial, in which healthy volunteers are given to assess whether it is toxicologically safe and whether it effectively induces an immune response. Clearly establishing the safety and efficacy of a vaccine may require phases 2, 2b and 3 of clinical studies, which takes more than a year. Additionally, NIAID is collaboratively with the University of Oxford in the UK developing another chimpanzee adenovirus vaccine, and are evaluating whether other vaccine candidates developed for SARS are effective for COVID-199.
As for therapeutics, NIAID itself is working collaboratively with the University of Nebraska in a randomized controlled clinical trial to evaluate the safety and efficacy of the antiviral drug remdesivir, in hospitalized patients diagnosed with COVID-19. Remdesivir is a broad-spectrum antiviral that is being evaluated in animal models to investigate its effectiveness in the treatment of MERS and SARS (Severe Acute Respiratory Syndrome)10. It is important to understand that undertaking these clinical studies, in addition to time, requires a high level of scientific, manufacturing and logistics capacity, independent ethical review, adequate regulation and large economic resources. A review of clinical study record bases as of March 24, 2020 shows that there are currently 536 relevant clinical studies, of which 332 are related to COVID-19. Among the latter 188 are open to recruit volunteers and 146 essays are prepared to recruit. The geographical distribution of these studies occurs mostly in China and Korea, as well as high-income countries in Europe and North America. Very few essays are planned for Central or South America, South or Southeast Asia or Africa11. Other therapies being evaluated are Kaletra, which is a combination of two HIV drugs: ritonavir and lopinavir; Kaletra plus interferon beta, which is a signal molecule that helps the immune system fight viruses, therefore is expected to help strengthen the action of drugs12; and hydroxychloroquine or chloroquine, which is an antimalarial that has sounded in recent weeks due to a small study in France with 20 patients13, but done without scientific rigor by being neither controlled nor randomized14.
WHO has recently announced the launch of the SOLIDARITY trial, a study of potential treatments for COVID-19 to be developed in Asia, South Africa, Europe and the Americas. Argentina is the first Latin American country to have confirmed its participation15.
In times of uncertainty, we can have peace of mind that science is the beacon that should guide our society and governments to make the best decisions and navigate this storm to safe harbor.
[1] WHO – Coronavirus Disease (COVID-19) Outbreak, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
[2] World of Molecules. https://www.worldofmolecules.com/coronavirus/how-soap-destroys-coronavirus.html
[3] Annals of Internal Medicine, 6 de Octubre de 2009. https://annals.org/aim/fullarticle/744899/facemasks-hand-hygiene-prevent-influenza-transmission-households-cluster-randomized-trial
[4] Journal of Hospital Infection, Mayo de 2013. https://www.sciencedirect.com/science/article/abs/pii/S0195670113000698
[5] Nature Medicine, 03 de Abril de 2020. https://www.nature.com/articles/s41591-020-0843-2
[6] The Lancet Infectious Diseases, 11 de Marzo de 2020. https://www.sciencedirect.com/science/article/pii/S1473309920301444
[7] Chest, 30 de Marzo de 2020. https://journal.chestnet.org/article/S0012-3692(20)30572-9/pdf
[8] Johns Hopkins COVID-19 Hub, 16 de Abril de 2020. https://hub.jhu.edu/2020/04/16/coronavirus-vaccine-timeline/
[9] NIAID, Abril de 2020. https://www.niaid.nih.gov/diseases-conditions/coronaviruses-therapeutics-vaccines
[10] NIAID, Abril de 2020. https://www.niaid.nih.gov/news-events/nih-clinical-trial-remdesivir-treat-covid-19-begins
[11] The Lancet, 02 de Abril de 2020. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30798-4/fulltext
[12] The New England Journal of Medicine, 18 de Marzo de 2020. https://www.nejm.org/doi/full/10.1056/NEJMoa2001282
[13] International Journal of Antimicrobial Agents, 20 de Marzo de 2020. https://www.sciencedirect.com/science/article/pii/S0924857920300996#bib0012
[14] International Society of Antimicrobial Chemotherapy, 03 de Abril de 2020. https://www.isac.world/news-and-publications/official-isac-statement
[15] OMS, 18 de marzo de 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---18-march-2020

















